
Nucleic acid extraction provides a foundation for many extensive research and applications (for example cloning, PCR analysis, and next-generation sequencing technology in the field of whole genome and transcriptome), and the obtained nucleic acid can be used in a variety of ways. Nucleic acid purification and quality assessment are important steps in experimental workflows, and the quality of nucleic acids can affect the performance in downstream reactions.
Streamlined protocols with optimized extraction reagents simplify handling and are optimized for various specimen types, formats, and throughputs, as well as for manual and automated processing.
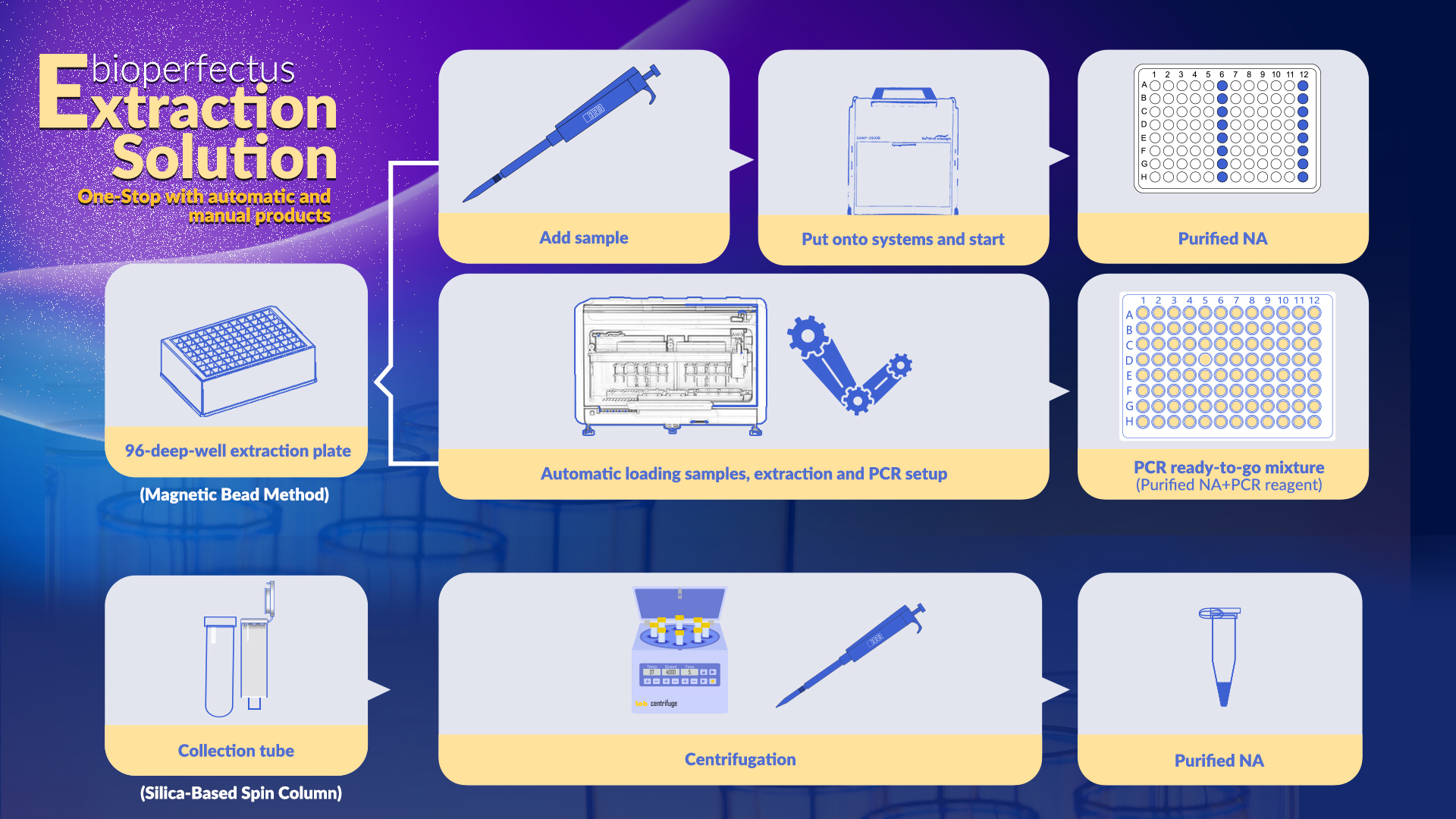
Bioperfectus provides nucleic acid extraction solutions, including systems and kits, which could satisfy the requirements of nucleic acid purification with multiple specimen types in automatic or manual ways.
Beyond SARS-COV-2, our extraction kits can be used to deal with more clinical samples to detect pathogens such as viruses, bacteria, and parasites. Thus we can provide extraction solutions for HPV, CT, NG, UU, Monkeypox virus, Adenovirus, Coxsackievirus, Influenza virus, Rubella virus, Measles virus, Dengue virus, Zika virus, Chikungunya virus, West Nile virus, Ebola virus and plasmodium parasites detection.

With the development of molecular analysis technology, extraction kits will be needed to purify nucleic acid efficiently, accurately, and economically. Bioperfectus extraction solution could provide products and services for 90% of clinical samples. In the future, we will launch more kits for more specimen types and offer a convenient way for purified nucleic acid and downstream analysis.

