As a manufacturer and innovator, we Bioperfectus are always on the way to go beyond testing.
We today are pleased to announce that our SARS-CoV-2 antigen rapid test kit has been upgraded to meet higher market requirements:
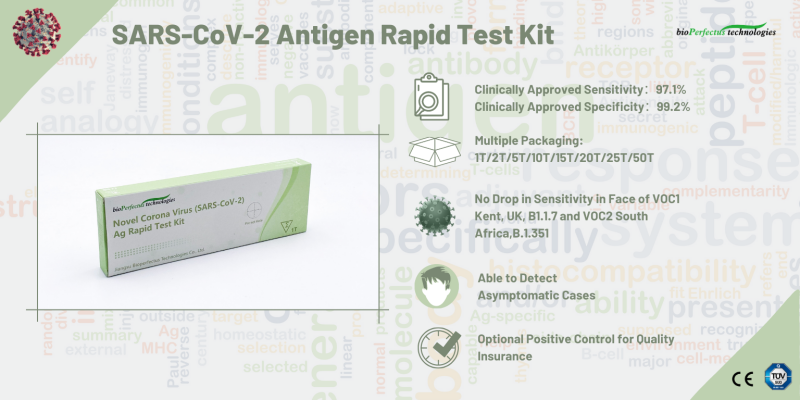
- Clinically approved sensitivity: 97.1%
- Clinically approved specificity: 99.2%
- Multiple packaging: 1T/2T/5T/10T/15T/20T/25T/50T
- No drop in sensitivity in face of VOC1 Kent, UK, B1.1.7 and VOC2 South Africa, B.1.351
- Able to detect asymptomatic cases
- Optional positive control for quality assurance
For more information about our product, please send an inquiry email to info@bioperfectus.com, media cooperation & news release: marketing_global@bioperfectus.com.