While the concern of the Delta variant infection has not faded away, our attentions were moved to the Lambda variant, which is now mainly widespread in South America.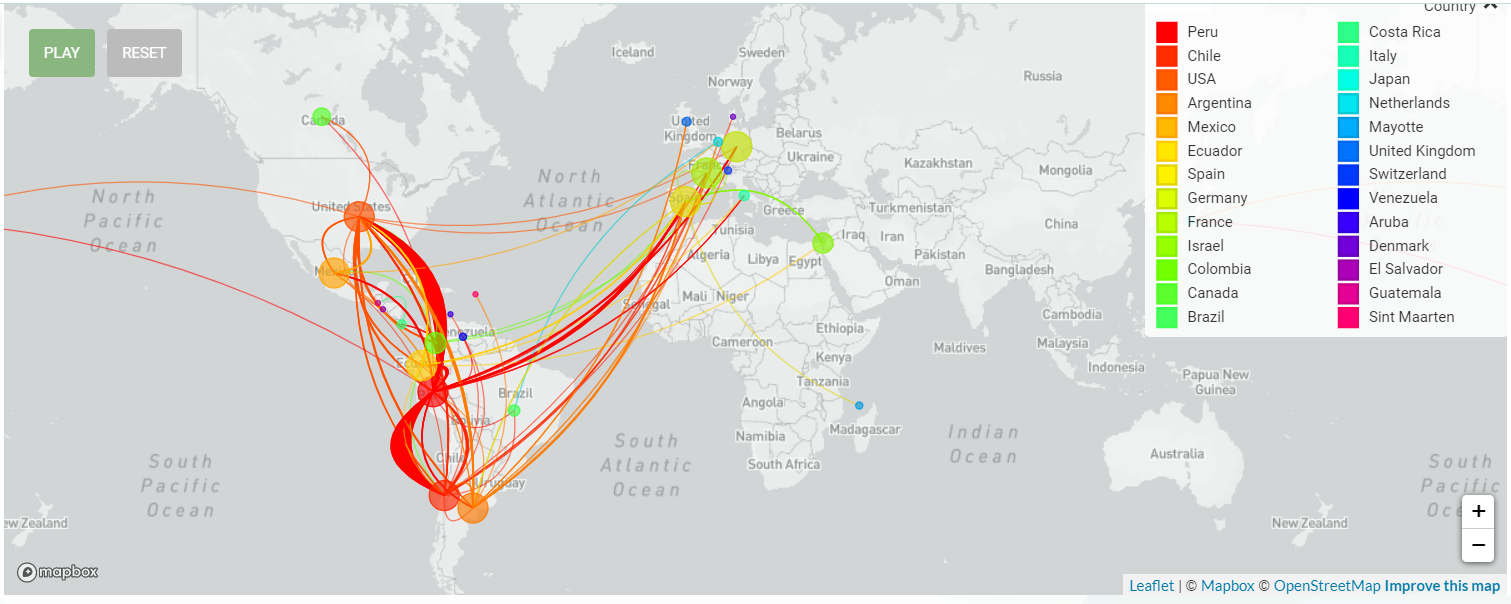
The Lambda variant (C.37) was first identified in Peru in 2020 and according to the recent shared sequencing data, the strain has been detected in at least 37 countries. Peru, Chile, Argentina and even in the 46 states of America were reported with high cumulative prevalence rate of Lambda variant. For now, the Lambda variant was designated as Variants of Interest for close surveillance.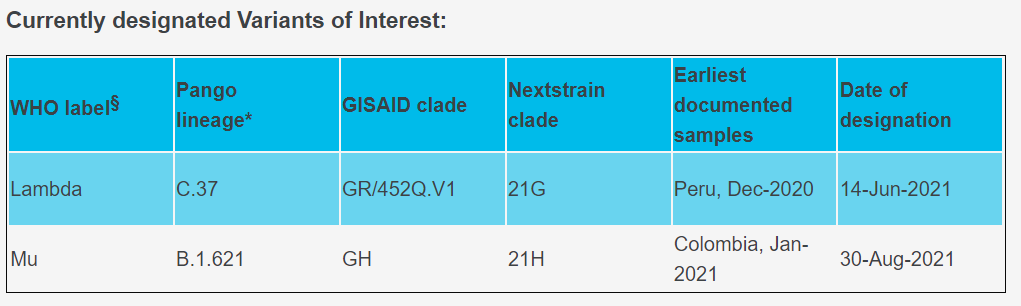
The Lambda variant was featured with increasing viral infectivity and exhibiting resistance to antiviral immunity. A study (Kimura et al.) has demonstrated that three mutations, the RSYLTPGD246-253N, L452Q and F490S mutations, respectively confer resistance to the vaccine-induced antiviral immunity. Additionally, the T76I and L452Q mutations also contribute to the enhanced viral infectivity.
We are very pleased to launch the new RUO product, SARS-CoV-2 Variant Lambda (C.37) Real Time PCR Kit (RUO), which will assist with the detection of the Lambda variant rapidly. This kit detects two of the highly prevalent mutation sites and identify Lambda variant by only one-tube RT-PCR reaction. With the automatic interpretation tool, the result can be interpretated directly right after the PCR program is finished.
For more information regarding variant detection kit for Delta and Lambda, contact us toady by email info@bioperfectus.com or marketing_global@bioperfectus.com.
References
- https://www.gisaid.org/hcov19-variants/
- SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance. Izumi Kimura, etc. The Genotype to Phenotype Japan (G2P-Japan) Consortium, Akatsuki Saito, So Nakagawa, Kei Sato. bioRxiv 2021.07.28.454085; doi: https://doi.org/10.1101/2021.07.28.454085

