The Mu variant (known as B.1.621), first identified in Colombia in January 2021, has been added to the WHO’s VOI list on 30 August. Until now it was detected in 39 countries and found to possess a cluster of mutations that might make it less susceptible to the immune protection, according to the WHO’s weekly bulletin on the pandemic.
Although the global prevalence of the variant among sequenced cases are currently below 0.1%, its prevalence in Colombia and Ecuador has consistently increased, where it accounts for 39% and 13% of Covid cases respectively.
Some concern aboutMucomes from the particular mutations it carries, such as the P681H, E484K etc., which may help the virus evade immunity defenses and transmit faster.
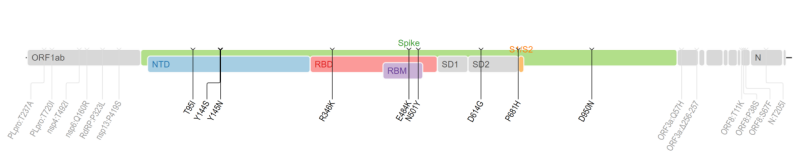
https://covdb.stanford.edu/page/mutation-viewer/#sec_b-1-621
Under the investigation of Bioperfectus R&D team, we hereby conclude thatBioperfectus COVID-19 Coronavirus Real Time PCR Kit, COVID-19 Coronavirus (ORF1ab/N/E) Real Time PCR Kit and COVID-19 Coronavirus and Influenza A/B Virus Real Time PCR Kit are confirmed to stay effective and free from the impact of the variant B.1.621.
Fact Sheet: Bioperfectus Products Free from the Impact of the VOCs & VOIs Declared by WHO*Updated by 2021/09/03
References:
https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-august-2021
https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/

