
With surging cases of prevailing SARS-CoV-2Deltavariant, the testing of the variant has become a necessary and important measure to curb the further spread of the virus.
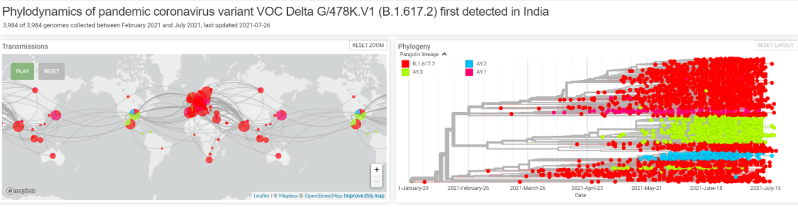
Source: GISAID, 2021,https://www.gisaid.org/hcov19-variants/
Under such circumstance, Bioperfectus, as a pioneer in the global molecular diagnostic market, is glad to launch our molecular RUO product to assist the detection of the SARS-CoV-2 variants.
Bioperfectus SARS-CoV-2 Variant B.1.617 Real Time PCR Kit is designed to detect L452R, E484Q, and P681R mutations in Sgene of SARS-CoV-2 and help to identify B.1.617.2 for research purpose.
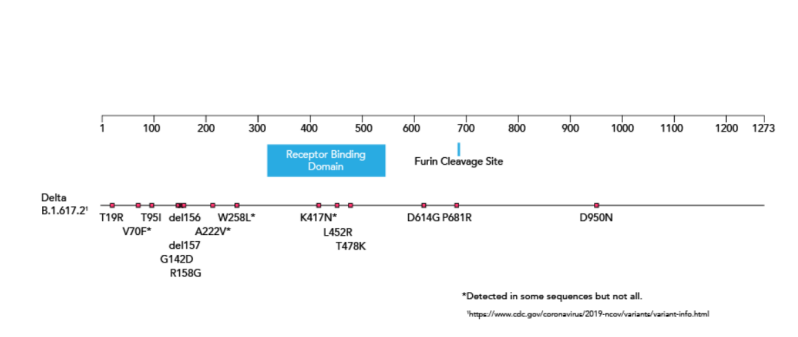
Source: American Society for Microbiology, July 23, 2021,https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2
Our R&D team is closely tracking the reported SARS-CoV-2 variants worldwide and design assays for detecting emerging mutations related to increased infectiousness, immune escape, vaccine efficacy, and therapeutic medicines. Assays for these emerging mutation targets of interest will be available upon request.
Learn more : https://www.bioperfectus.com/productinfo/668939.html
Inquiry email : info@bioperfectus.com,marketing_global@bioperfectus.com

