HFMD is a significant health and economic burden for the Asia Pacific region. It has been estimated that the average direct and indirect cost was US$129 for an outpatient, $484 for an inpatient, and $1936 for a severe case of HFMD, respectively.
Hand, foot, and mouth disease (HFMD) is a common infectious disease in children caused by a group of viruses from the genus Enterovirus and which is principally seen in children under 5 years of age. The symptom is characterized by fever, mouth sores, and blisters on the hands, feet, and buttocks. Severe forms of the disease characterized by severe complications due to the dissemination of the disease at neurological, cardiovascular, or respiratory levels can cause the death of children. In most cases, HFMD is a self-limiting illness, with the majority of children recovering spontaneously with symptomatic treatment. Only a tiny proportion of children with HFMD develop neurological involvement, which may further progress to potentially fatal cardiopulmonary failure.
The transmission route is spread through direct contact with the nasal discharge fluid from blisters, saliva, or feces of an infected person. Outbreaks can occur from spring to fall, and most cases occur in patients younger than 10 years old. The incubation period for HFMD is 3 to 7 days.
The primary pathogens responsible for the disease include enteroviruses in the coxsackievirus (CV) group A, type 4-7, 9, 10, and 16, as well as group B types 1-3, and 5, some echovirus serotypes, and enterovirus A71 (EV-A71).
About coxsackievirus
- The first coxsackievirus was isolated only in 1948 in New York state (USA) by Dalldorf et al, who investigated an outbreak of paralytic poliomyelitis in the village of Coxsackie.
- Coxsackieviruses belong to the Picornaviridae family, Enterovirus genus, group of human enteroviruses.
- Positive-sense single-stranded RNA viruses protected by an icosahedral capsid exclusively constituted of proteins.
- The viral particle contains no enzyme and no envelope. Its size is 22-30 nm.
- Divided into two subgroups A and B: coxsackievirus A (CV-A) with 23 serotypes 1-24 (23 is missing) and coxsackievirus B (CV-B) with six serotypes 1-6. (This classification is based on the histopathologic lesions in suckling mice.)
Coxsackievirus A:
- Usually affects the skin and mucous membranes.
- Causes herpangina and HFMD.
- The most common causes of HFMD are Coxsackievirus A16 (CA16) along with the closely related enterovirus 71 (EV71).
Coxsackievirus B:
- Usually affects the heart, lungs, pancreas, and liver.
- Causes Bornholm disease, hepatitis, myocarditis, and pericarditis.
- Heart Conditions such as Pericarditis and Myocarditis.
The most common causes of HFMD are CV-A16 and EV-A71, while CV-A6 and CV-A10 case burden has increased significantly in some areas in recent years. Similar to the CV-A16/EV-A71 group, CV-A6 and CV-A10 often co-circulate. According to some recent studies, EV-A71, EV-A16, EV-A6, and EV-A10 are the most commonly sequenced types in East Asia and Southeast Asia. Coxsackieviruses CV-A6 and CV-A10 have been mainly associated with HA outbreaks and, recently, HFMD epidemics. CV-A6 tends to be a virulent strain that unusually affects both pediatric and adult populations. The virus has been responsible for severe atypical cases of HFMD, often requiring hospitalization, characterized by extensive vesiculobullous and erosive cutaneous eruptions, eczema, purpuric lesions, and onychomadesis with nail shedding. CV-A6 infections have also led to fatal encephalitis/encephalopathy and myocarditis. CV-A10-associated HFMD cases are characterized by high fever, vesicular rashes, and oral ulcers with occasional meningoencephalitis complications.
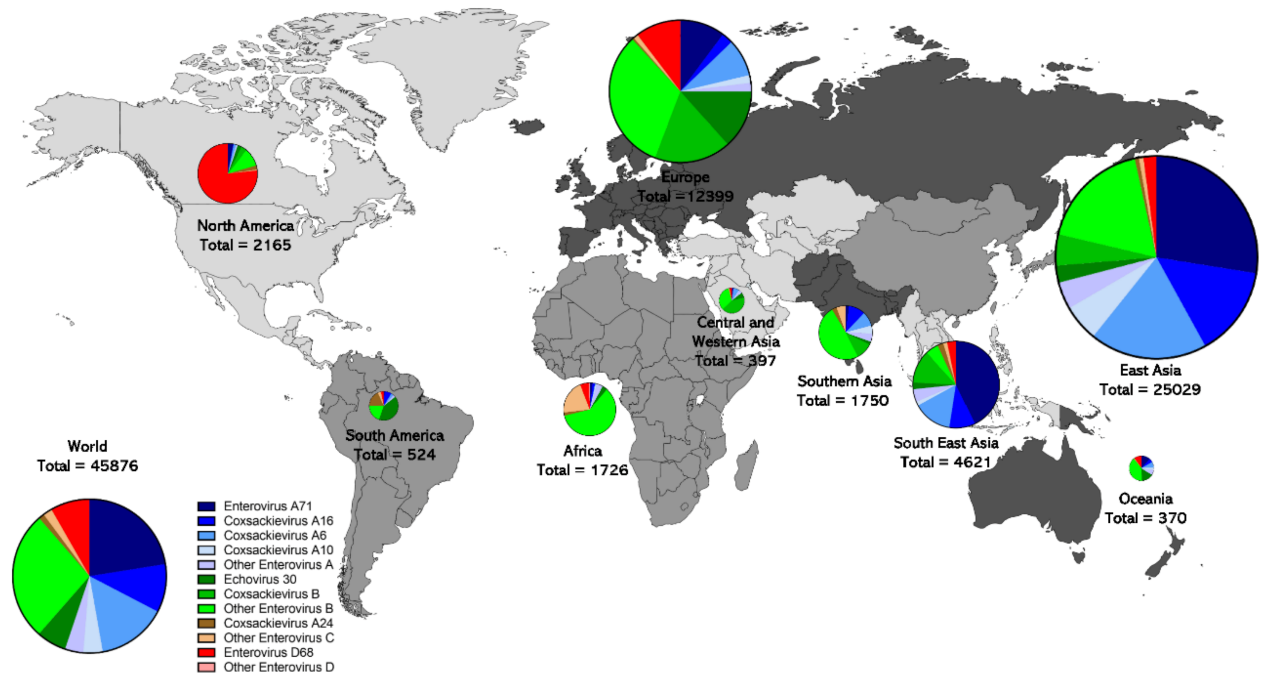
Geographic distribution of non-polio enterovirus genomic sequence records.
To diagnose HFMD, clinicians primarily rely on physical symptoms. Since the symptoms of HFMD overlap with many other illnesses, including chicken pox, oral herpes (cold sores), eczema, etc., and may lead to patients being administered incorrect treatment and further delays in recovery. It may also increase the likelihood of transmission if patients are not promptly isolated. Identification of the agents responsible for outbreaks of HFMD is also essential, which will help predict the outbreak’s severity and initiate appropriate public health interventions.
The different EV-A types exhibit different antigenic properties, and there is no protective cross-immunity between the different enteroviruses types. Another enterovirus type almost always causes recurrent HFMD. There is no multivalent HFMD vaccine available; only an EV-A71 vaccine has been available in China since 2015. The increasing prevalence of CV-A6 and CV-A10 suggests the need for a HFMD vaccine strategy that includes those EV types besides CV-A16, especially considering the incidence of severe HFMD attributed to CV-A6.
Today we are very pleased to introduce our CE-marked product Coxsackievirus A6 and A10 Real-Time PCR Kit to the global market. This kit can simultaneously detect and distinguish CA6 and CA10 in one test from throat swabs. With the benefit of Real-time PCR technology, the result can be received quickly with good sensitivity and high specificity with no cross-reaction with other similar pathogens. This kit will help diagnose and identify the agents quickly and help to provide surveillant information which will greatly help develop a vaccine strategy.
References:
- Klein M, Chong P. Is a multivalent hand, foot, and mouth disease vaccine feasible? Hum Vaccin Immunother. 2015;11(11):2688-704. doi: 10.1080/21645515.2015.1049780. Epub 2015 May 26. PMID: 26009802; PMCID: PMC4685682.
- Li, XW., Ni, X., Qian, SY. et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr 14, 437–447 (2018). https://doi.org/10.1007/s12519-018-0189-8.
- Brown, D.M.; Zhang, Y.; Scheuermann, R.H. Epidemiology and Sequence-Based Evolutionary Analysis of Circulating Non-Polio Enteroviruses. Microorganisms 2020, 8, 1856. https://doi.org/10.3390/microorganisms8121856

