In April 2025, the American Society for Colposcopy and Cervical Pathology (ASCCP) officially released "Applying Results of Extended Genotyping to Management of Positive Cervicovaginal Human Papillomavirus Test Results: Enduring Guidelines". The Enduring Consensus Cervical Cancer Screening and Management Guidelines Committee developed recommendations for the use of extended genotyping results in cervical cancer prevention programs. The specific content is interpreted as follows:

Essentially all cervical cancers are caused by infection with carcinogenic types of the human papillomavirus (HPV). Detection of HPV or HPV-induced cytologic changes allows for triage to colposcopy for detection of cancer precursors, whose treatment reduces cervical cancer risk.
Human papillomavirus types vary widely in carcinogenicity. The International Agency for Research on Cancer (IARC), in collaboration with international experts, has established a hierarchy of HPV genotypes based on worldwide epidemiologic data. Table 1 summarizes cervical cancer attribution, that is, the proportion of cancers caused by specific HPV types, and risk of progression from infection to CIN3+, for 13 types (12 carcinogenic types and 1 probable carcinogenic type). The IARC combines carcinogenic HPV types into 4 groups based on their risk of progression and attribution to cancer: HPV16, HPV18/45, HPV16-related types (HPV33, 31, 52, 58, 35), and the remaining other carcinogenic or probable carcinogenic types (HPV 39, 51, 59, 56, 68). However, the clinical management of HPV types and type groups is not prescribed by the IARC ranking and may vary across settings, depending on specific risk-based thresholds and available triage options, as well as on colposcopy and treatment
capacity.
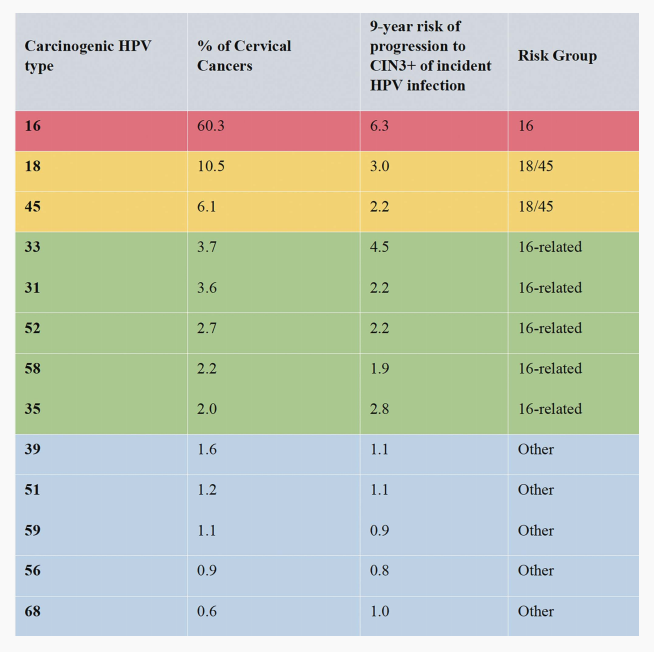
TABLE 1. Contribution of Carcinogenic Human Papillomavirus (HPV) Genotypes and CIN3+ Progression Risk for Progression to Cervical Intraepithelial Neoplasia (CIN) Grade 3 or Worse
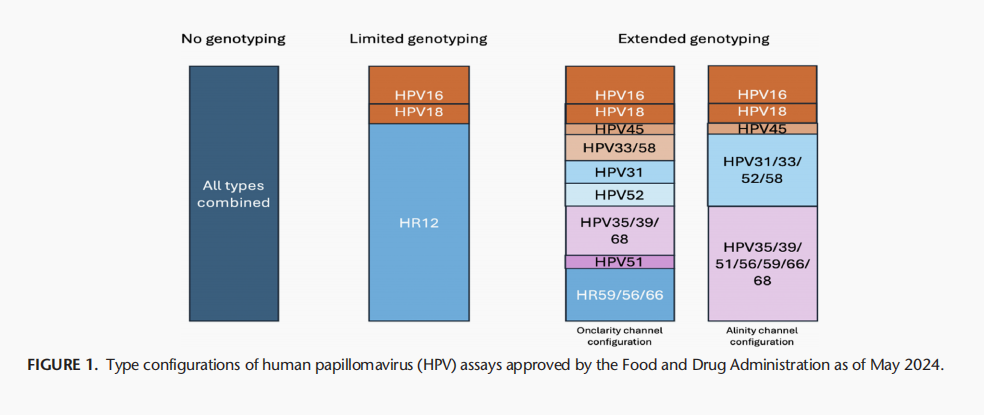
Recently, the US Food and Drug Administration (FDA) approved 2 assays that provide extended genotyping: the Onclarity HPVAssay (Becton Dickinson, Franklin Lakes, NJ, approved April 2020) identifies HPV types 16, 18, 45, 31, 51, and 52 in individual assay channels and reports assay channels combining HPV33/58, HPV35/39/68, and HPV56/59/66; and the Alinity m High-Risk HPV Assay (Abbott, Abbott Park, IL, approved November 2023) identifies HPV types 16, 18, and 45 individually and reports combined types 31/33/52/58 and 35/39/51/56/59/66/68.
Recommendation terminology (recommended, preferred, acceptable, not recommended), recommendation strength (A–E), and quality of evidence (I–III) followed that of the 2019 Guidelines. Several key points apply when employing recommendations for extended genotyping:
1. Recommendations only apply to FDA-approved extended genotyping assays. At the time of writing, the BD Onclarity HPV Assay and the Abbott Alinity m High-Risk HPV Assay were the only extended genotyping tests with FDA approval.
2. These recommendations apply only to results obtained in asymptomatic individuals with cervices undergoing screening or surveillance. Symptomatic patients should be managed according to relevant protocols. Screening after hysterectomy should be limited to individuals with prior history of CIN2+ or cervical cancer; risks for those individuals are undetermined, and these guidelines may not apply.
ECTION 1: GENERAL PRINCIPLES
Recommendation #1: HPV Extended Genotyping Is Acceptable to Guide Clinical Management in the Setting of a Positive HPV Test Result. (BII)
Recommendation #2: When Multiple Types Are Reported, Management According to the Type With Highest Cancer Risk Is Recommended Following the IARC Hierarchy 16, 18, 45, 33, 31, 52, 58, 35, 39, 51, 59, 56, 68, 66. (BII)
SECTION 2: ASSAY- AND GENOTYPESPECIFIC RECOMMENDATIONS
Previous Recommendation for HPV16 and HPV18
When primary HPV screening is used, performance of an additional reflex triage test (eg, reflex cytology) for all positive HPV tests regardless of genotype is preferred (this includes tests positive for genotypes HPV 16/18) (CIII). However, if primary HPV screening test genotyping results are HPV16- or HPV18-positive and reflex triage testing from the same laboratory specimen is not feasible, referral for colposcopy prior to obtaining additional testing is acceptable (CIII). If genotyping for HPV 16 or HPV 18 is positive, and triage testing is not performed prior to the colposcopy, collection of an additional triage test (eg, reflex cytology) at the colposcopy visit is recommended (CIII)
New Recommendations for Types Other Than HPV16 and HPV 18
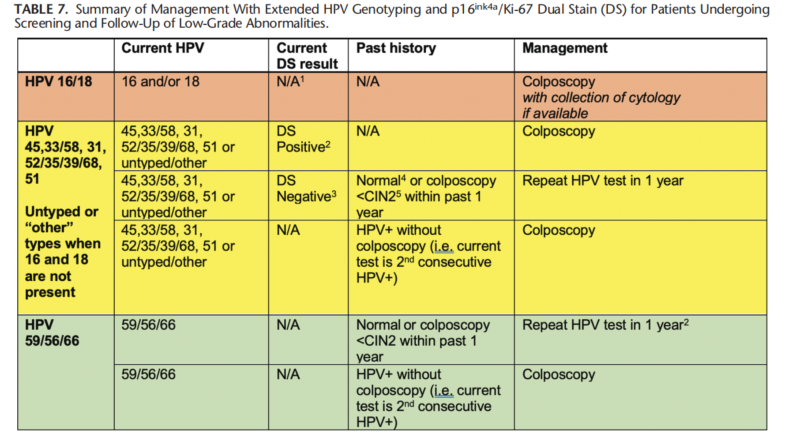
Recommendation #3: In a Screening Setting Using Either Primary HPV Testing or Cotesting, for Patients Who Test Positive for HPV 31, 33/58, 35/39/68, 45, 51, 52, or Combinations Thereof, But Negative for HPV16 and HPV18, Triage Testing With Dual Stain or Cytology Is Recommended. If Results Are Dual Stain-Negative or NILM Cytology, Repeat HPV testing in 1 year Is Recommended. If Results Are Dual Stain-Positive or Cytology ASC-US, LSIL, ASC-H, AGC, HSIL, or Carcinoma, Colposcopy Is Recommended. (AII) for Patients With Initial Results of Dual Stain-Negative or NILM Cytology Who Undergo Repeat HPV Testing or Cotesting at
1 Year, if Results Are HPV-Positive for Any HPV Type or if Cytology of ASC-H, AGC, HSIL, or Carcinoma, Colposcopy Is Recommended. (CIII). If Results Are HPV-Negative But Cytology Is NILM, ASC-US, or LSIL, HPV-Based Testing in 12 Months Is Recommended. (CIII)
HPV 35/39/68.
According to the IARC carcinogenicity ranking, HPV35 is grouped with HPV16-related types, whereas HPV39 and HPV68 are in the lower risk group. For patients with HPV35/39/68, we found differential immediate CIN3+ risks in the IRIS cohort (HPV35/39/68: 1.7%) versus the majority African American STRIDES cohort (HPV35/39/68: 5.9%), consistent with the higher prevalence and cancer attribution of HPV35 in those of African descent.19 To ensure that individuals with HPV35 infections undergo appropriate triage, the HPV35/39/68 channel should be managed like the other HPV16-related types.
HPV 51.
Immediate CIN3+ risks also differed for those with HPV51 in the IRIS cohort (1.5%) and STRIDES cohort (6.3%). Since the number of HPV51 infections with CIN3+ outcomes was limited, the evidence was insufficient to escalate management, so the Enduring Guidelines Working Group applied the 2019 recommendation for non-16/18 HPV types: to perform a triage test.
HPV 31.
Risk analyses from the IRIS and STRIDES cohorts suggested that HPV31 can be successfully triaged with cytology or dual stain, supporting a triage recommendation. This is also the current standard for HR12. In contrast, the Onclarity regulatory trial data reported that individuals with HPV31 and negative cytology results had a 7.5% risk of CIN3+, which would support immediate referral to colposcopy. This finding was not confirmed in other larger studies. Therefore, the nduring Guidelines group considered evidence insufficient to change current practice.
HPV 45.
The utility of triage was assessed for HPV45. Human papillomavirus 45, like HPV18, is associated with increased risk of adenocarcinoma and its precursor, AIS. Both cytology and dual stain triaged HPV45 with high sensitivity for CIN3+. This high sensitivity for triage testing supported grouping HPV45 with the intermediate-risk carcinogenic HPV group managed with triage.
Recommendation #4: In a Screening Setting Using Primary HPV Testing, for Patients Who Test Positive for HPV Types 56/59/66 and No Other Carcinogenic Types, Repeat HPV Testing in 1 Year Is Recommended. (AII) If HPV-Positive for Any HPV Type at the 1-Year Follow-Up, Colposcopy Is Recommended. (CIII)
Recommendation #5: In a Screening Setting Using Cotesting, for Patients Who Test Positive for HPV Types 56/59/66 and No Other Carcinogenic Types, 1-Year Return Is Recommended for NILM, ASC-US, and LSIL, and Colposcopy Is Recommended for ASC-H, AGC, HSIL, or Carcinoma. (AII) At the 1-Year Follow-Up, Colposcopy Is Recommended if HPV Results Are Positive for Any HPV Type or if Cytology Results Are ASC-H, AGC, HSIL, or Carcinoma. (CIII)
Recommendation #6: During Surveillance, When Patients Are Being Followed Who Had No Preceding High-Grade Cytology (ASC-H, AGC, HSIL, or Carcinoma) or Histology (CIN2, CIN3, or AIS), Use of Extended Genotyping Results According to the Guidelines Outlined for Screening Is Acceptable. (CIII) When High-Grade Cytology or Histology Results Are Present and in the Posttreatment Setting, Management Using the 2019 Guidelines Is Recommended. (CIII)
Reference: Applying Results of Extended Genotyping to Management of Positive Cervicovaginal Human Papillomavirus Test Results: Enduring Guidelines. J Low Genit Tract Dis. 2025 Apr 1;29(2):134-143.

