Recently, Dr. Zuyi Chen’s team from the School of Laboratory Medicine at Zunyi Medical University and the Department of Clinical Laboratory at the Affiliated Hospital of Zunyi Medical University published a research article in Virology Journal titled “Study on the clinical characteristics, persistent infection capability, and viral load of human papillomavirus type 82 single infection.” The study investigates the positivity rate and clinical features of single HPV82 infection, as well as the relationship between viral load, persistence, and pathogenicity.

Conclusion
HPV82 is a rare high-risk HPV type. Its pathogenic potential from a single infection ranks in the middle among high-risk types and can lead to cervical cancer. A high proportion of CIN3 cases was observed in HPV82 single infections. HPV82 should be included in the expanded screening for HPV. High viral load is a significant factor that improves the persistent infection ability and pathogenicity of HPV82. Viral load is expected to serve as a screening risk factor for persistent infection and disease progression associated with HPV82.
Methods
Women who underwent gynecological examinations or sought medical care at the hospital between January 1, 2014 and December 31, 2023 were included in the study. HPV testing was conducted, followed by pathological examinations, re-evaluations, and follow-up investigations.
HPV Testing
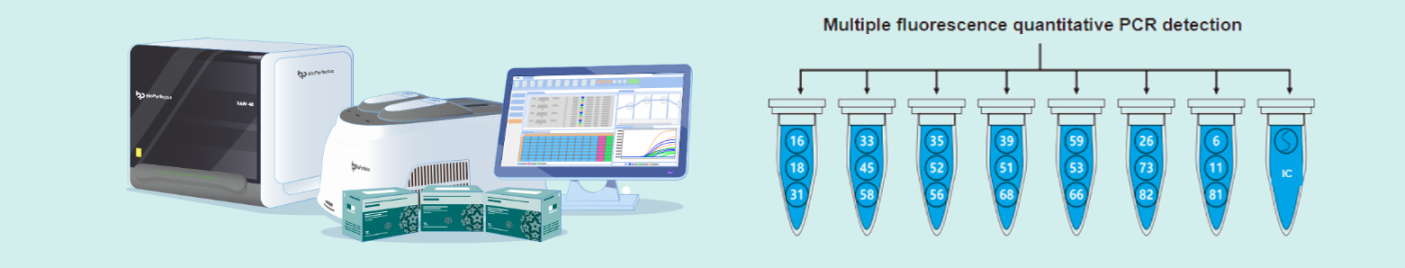
The human papillomavirus nucleic acid genotyping detection kit (fluorescent PCR method) (National Medical Device Registration Certificate No. 20153400364, Jiangsu BioPerfectus Co., China, detection limit: 20 copies/reaction, specificity: 99.6%. catalog number: JC80301) was used to determine the viral load of HPV82. This kit employs real-time fluorescent PCR to simultaneously quantify the HPV genome and human housekeeping genes in different reaction tubes . The HPV82 viral load was calculated per 10,000 cells based on the concentration of the housekeeping genes.
Pathological examination
HPV82 single infection were selected for the cross-sectional study, with the following exclusion criteria: human immunodeficiency virus infection, use of immunosuppressive medications, or refusal of tissue pathological biopsy. After gynecological colposcopy or pathological ThinPrep™ Pap Test PreservCyt (TCT) examination, patients with abnormalities underwent histopathological examination to distinguish between lesion-free tissue, cervical intraepithelial neoplasia (CIN) grades 1–3, and cervical cancer.
Follow-up Study
HPV82-positive women were advised to undergo re-examination and HPV retesting every six months from the initial examination, with follow-up lasting 6–24 months. The exclusion criteria for follow-up were cervical surgery, loss to follow-up, or concurrent high-risk infections.
Results
HPV82 positivity rate
Among women undergoing gynecological physical examinations or seeking medical consultation, the HPV82 positivity rate was approximately 0.24% (1,033/435,072). Among the HPV types detected, the detection rate of HPV82 was 1.00% (1,033/103,608). Of the 1,033 women infected with HPV82, 342 had a single infection.
Among all the detected types, the detection rates of high-risk types were as follows: HPV52 (18.07%), 16 (11.89%), 58 (10.67%), 53 (8.48%), 39 (6.48%), 51 (5.13%), 68 (4.59%), 56 (3.90%), 18 (3.74%), 33 (3.54%), 66 (3.20%), 59 (2.96%), 31 (2.65%), 35 (1.24%), 82 (1.00%), 45 (0.91%), 73 (0.59%), 26 (0.37%). The detection rate of HPV82 ranked fifteenth among the high-risk types.

Cross-sectional study results
A total of 335 participants were included in the cross-sectional study (mean age 39.74 ± 10.53 years, age range 22–74 years). Among the 335 cases of HPV82 single infection, histopathological biopsy results showed that 263 patients were lesion-free, 42 had cervical intraepithelial neoplasia grade 1 (CIN1), 11 had CIN2, 18 had CIN3, and 1 patient was diagnosed with cervical cancer.
Follow-up results
A total of 210 patients completed follow-up (mean duration 12.46 ± 3.25 months, range 6.90–22.73 months). Among the 33 patients with CIN1, 21.21% (7/33) progressed to more severe cervical lesions. In contrast, among the 177 lesion-free patients, 7.34% (13/177) progressed to CIN. The progression rate in CIN1 patients (21.21%, 7/33) was significantly higher than that in lesion-free patients (7.34%, 13/177) (relative risk = 2.89, 95% confidence interval = 1.25–6.69, χ² = 6.21, p = 0.013).
Viral load comparison
The viral load in the CIN and cervical cancer groups was significantly higher than that in the lesion-free group (p < 0.001), and the viral load in the persistent infection group was higher than that in the viral clearance group (p < 0.001):
The mean logarithmic value of viral load (units: log10 copies per 10,000 cells) in patients with cervical cancer and CIN was significantly higher (4.41) than that in the lesion-free group (3.90) (t = 3.79, p < 0.001).
In the follow-up phase, the average log viral load in the progression group (CIN1 progressing to CIN2, lesion-free progressing to CIN1 or CIN2) (4.53) was significantly higher than that in the maintenance and regression group (3.79) (t = 3.11, p = 0.002).
Furthermore, the log viral load of the persistent HPV82 infection group (4.53) was significantly higher than that of the non-persistent infection group (3.79) (t = 4.70, p < 0.001).
BioPerfectus Quantitative HPV Genotyping Detection System
BioPerfectus Quantitative HPV Genotyping Detection System utilizes fluorescent quantitative PCR technology and employs a “compartmentalized typing, closed-tube detection” approach to achieve precise identification of 21 HPV subtypes. At the same time, by detecting the human single-copy TOP3 gene, it calibrates the number of sampled cells to eliminate variability due to differing cell counts. This enables the calculation of viral copy numbers of each HPV genotype per 10,000 cells, achieving precise HPV typing alongside standardized quantitative detection.